THE WORLD OF ATOMS
Most people have heard of the oriental race which puzzled over the foundations of the universe, and decided that it must be supported on the back of a giant elephant. But the elephant? They put it on the back of a monstrous tortoise, and there they let the matter end. If every animal in nature had been called upon, they would have been no nearer a foundation. Most ancient peoples, indeed, made no effort to find a foundation. The universe was a very compact little structure, mainly composed of the earth and the great canopy over the earth which they called the sky. They left it, as a whole, floating in nothing. And in this the ancients were wiser than they knew. Things do not fall down unless they are pulled down by that mysterious force which we call gravitation. The earth, it is true, is pulled by the sun, and would fall into it; but the earth escapes this fiery fate by circulating at great speed round the sun. The stars pull each other; but it has already been explained that they meet this by travelling rapidly in gigantic orbits. Yet we do, in a new sense of the word, need foundations of the universe. Our mind craves for some explanation of the matter out of which the universe is made. For this explanation we turn to modern Physics and Chemistry. Both these sciences study, under different aspects, matter and energy; and between them they have put together a conception of the fundamental nature of things which marks an epoch in the history of human thought.[Pg 246]
§ 1
The Bricks of the Cosmos
More than two thousand years ago the first men of science, the Greeks of the cities of Asia Minor, speculated on the nature of matter. You can grind a piece of stone into dust. You can divide a spoonful of water into as many drops as you like. Apparently you can go on dividing as long as you have got apparatus fine enough for the work. But there must be a limit, these Greeks said, and so they supposed that all matter was ultimately composed of minute particles which were indivisible. That is the meaning of the Greek word "atom."
Like so many other ideas of these brilliant early Greek thinkers, the atom was a sound conception. We know to-day that matter is composed of atoms. But science was then so young that the way in which the Greeks applied the idea was not very profound. A liquid or a gas, they said, consisted of round, smooth atoms, which would not cling together. Then there were atoms with rough surfaces, "hooky" surfaces, and these stuck together and formed solids. The atoms of iron or marble, for instance, were so very hooky that, once they got together, a strong man could not tear them apart. The Greeks thought that the explanation of the universe was that an infinite number of these atoms had been moving and mixing in an infinite space during an infinite time, and had at last hit by chance on the particular combination which is our universe.
This was too simple and superficial. The idea of atoms was cast aside, only to be advanced again in various ways. It was the famous Manchester chemist, John Dalton, who restored it in the early years of the nineteenth century. He first definitely formulated the atomic theory as a scientific hypothesis. The whole physical and chemical science of that century was now based upon the atom, and it is quite a mistake to suppose that recent discoveries have discredited "atomism." An atom is the smallest particle[Pg 247] of a chemical element. No one has ever seen an atom. Even the wonderful new microscope which has just been invented cannot possibly show us particles of matter which are a million times smaller than the breadth of a hair; for that is the size of atoms. We can weigh them and measure them, though they are invisible, and we know that all matter is composed of them. It is a new discovery that atoms are not indivisible. They consist themselves of still smaller particles, as we shall see. But the atoms exist all the same, and we may still say that they are the bricks of which the material universe is built.
Photo: Elliott & Fry.
SIR ERNEST RUTHERFORD
One of our most eminent physicists who has succeeded Sir J. J. Thomson as Cavendish Professor of Physics at the University of Cambridge. The modern theory of the structure of the atom is largely due to him.

Photo: Rischgitz Collection.
J. CLERK-MAXWELL
One of the greatest scientific men who have ever lived. He revolutionised physics with his electro-magnetic theory of light, and practically all modern researches have had their origin, direct or indirect, in his work. Together with Faraday he constitutes one of the main scientific glories of the nineteenth century.

Photo: Ernest H. Mills.
SIR WILLIAM CROOKES
Sir William Crookes experimented on the electric discharge in vacuum tubes and described the phenomena as a "fourth state of matter." He was actually observing the flight of electrons, but he did not fully appreciate the nature of his experiments.

Photo: Photo Press
PROFESSOR SIR W. H. BRAGG
One of the most distinguished physicists of the present day.

But if we had some magical glass by means of which we could see into the structure of material things, we should not see the atoms put evenly together as bricks are in a wall. As a rule, two or more atoms first come together to form a larger particle, which we call a "molecule." Single atoms do not, as a rule, exist apart from other atoms; if a molecule is broken up, the individual atoms seek to unite with other atoms of another kind or amongst themselves. For example, three atoms of oxygen form what we call ozone; two atoms of hydrogen uniting with one atom of oxygen form water. It is molecules that form the mass of matter; a molecule, as it has been expressed, is a little building of which atoms are the bricks.
In this way we get a useful first view of the material things we handle. In a liquid the molecules of the liquid cling together loosely. They remain together as a body, but they roll over and away from each other. There is "cohesion" between them, but it is less powerful than in a solid. Put some water in a kettle over the lighted gas, and presently the tiny molecules of water will rush through the spout in a cloud of steam and scatter over the kitchen. The heat has broken their bond of association and turned the water into something like a gas; though we know that the particles will come together again, as they cool, and form once more drops of water.
In a gas the molecules have full individual liberty. They[Pg 248] are in a state of violent movement, and they form no union with each other. If we want to force them to enter into the loose sort of association which molecules have in a liquid, we have to slow down their individual movements by applying severe cold. That is how a modern man of science liquefies gases. No power that we have will liquefy air at its ordinary temperature. In very severe cold, on the other hand, the air will spontaneously become liquid. Some day, when the fires of the sun have sunk very low, the temperature of the earth will be less than -200° C.: that is to say, more than two hundred degrees Centigrade below freezing-point. It will sink to the temperature of the moon. Our atmosphere will then be an ocean of liquid air, 35 feet deep, lying upon the solidly frozen masses of our water-oceans.
In a solid the molecules cling firmly to each other. We need a force equal to twenty-five tons to tear asunder the molecules in a bar of iron an inch thick. Yet the structure is not "solid" in the popular sense of the word. If you put a piece of solid gold in a little pool of mercury, the gold will take in the mercury between its molecules, as if it were porous like a sponge. The hardest solid is more like a lattice-work than what we usually mean by "solid"; though the molecules are not fixed, like the bars of a lattice-work, but are in violent motion; they vibrate about equilibrium positions. If we could see right into the heart of a bit of the hardest steel, we should see billions of separate molecules, at some distance from each other, all moving rapidly to and fro.
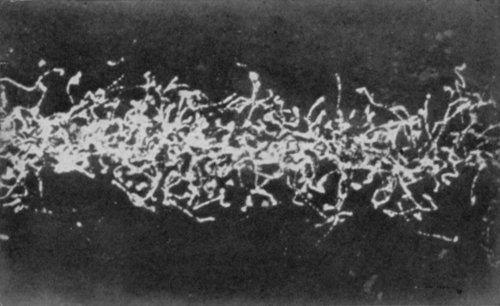
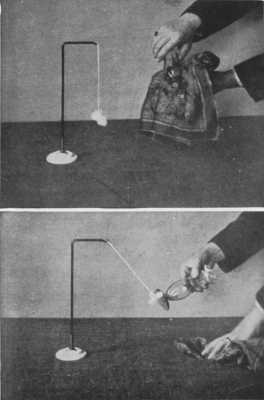
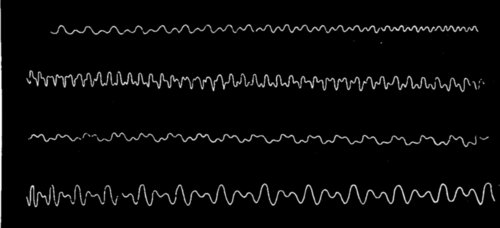
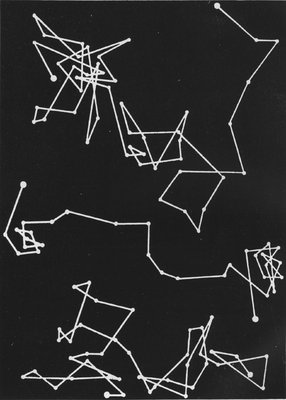
This molecular movement can, in a measure, be made visible. It was noticed by a microscopist named Brown that, in a solution containing very fine suspended particles, the particles were in constant movement. Under a powerful microscope these particles are seen to be violently agitated; they are each independently darting hither and thither somewhat like a lot of billiard balls on a billiard table, colliding and bounding about in all directions. Thousands of times a second these encounters occur, and this lively commotion is always going on, this incessant colliding of[Pg 249] one molecule with another is the normal condition of affairs; not one of them is at rest. The reason for this has been worked out, and it is now known that these particles move about because they are being incessantly bombarded by the molecules of the liquid. The molecules cannot, of course, be seen, but the fact of their incessant movement is revealed to the eye by the behaviour of the visible suspended particles. This incessant movement in the world of molecules is called the Brownian movement, and is a striking proof of the reality of molecular motions.
§ 2
The Wonder-World of Atoms
The exploration of this wonder-world of atoms and molecules by the physicists and chemists of to-day is one of the most impressive triumphs of modern science. Quite apart from radium and electrons and other sensational discoveries of recent years, the study of ordinary matter is hardly inferior, either in interest or audacity, to the work of the astronomer. And there is the same foundation in both cases—marvellous apparatus, and trains of mathematical reasoning that would have astonished Euclid or Archimedes. Extraordinary, therefore, as are some of the facts and figures we are now going to give in connection with the minuteness of atoms and molecules, let us bear in mind that we owe them to the most solid and severe processes of human thought.
Yet the principle can in most cases be made so clear that the reader will not be asked to take much on trust. It is, for instance, a matter of common knowledge that gold is soft enough to be beaten into gold leaf. It is a matter of common sense, one hopes, that if you beat a measured cube of gold into a leaf six inches square, the mathematician can tell the thickness of that leaf without measuring it. As a matter of fact, a single grain of gold has been beaten into a leaf seventy-five inches square. Now the mathematician can easily find that when a single grain of gold is beaten out to that size, the leaf must be 1/367,000 of an inch thick,[Pg 250] or about a thousand times thinner than the paper on which these words are printed; yet the leaf must be several molecules thick.
The finest gold leaf is, in fact, too thick for our purpose, and we turn with a new interest to that toy of our boyhood the soap-bubble. If you carefully examine one of these delicate films of soapy water, you notice certain dark spots or patches on them. These are their thinnest parts, and by two quite independent methods—one using electricity and the other light—we have found that at these spots the bubble is less than the three-millionth of an inch thick! But the molecules in the film cling together so firmly that they must be at least twenty or thirty deep in the thinnest part. A molecule, therefore, must be far less than the three-millionth of an inch thick.
We found next that a film of oil on the surface of water may be even thinner than a soap-bubble. Professor Perrin, the great French authority on atoms, got films of oil down to the fifty-millionth of an inch in thickness! He poured a measured drop of oil upon water. Then he found the exact limits of the area of the oil-sheet by blowing upon the water a fine powder which spread to the edge of the film and clearly outlined it. The rest is safe and simple calculation, as in the case of the beaten grain of gold. Now this film of oil must have been at least two molecules deep, so a single molecule of oil is considerably less than a hundred-millionth of an inch in diameter.
Innumerable methods have been tried, and the result is always the same. A single grain of indigo, for instance, will colour a ton of water. This obviously means that the grain contains billions of molecules which spread through the water. A grain of musk will scent a room—pour molecules into every part of it—for several years, yet not lose one-millionth of its mass in a year. There are a hundred ways of showing the minuteness of the ultimate particles of matter, and some of these enable us to give definite figures. On a careful comparison of the best methods we can say that the average molecule of matter is less[Pg 251] than the 1/125,000,000 of an inch in diameter. In a single cubic centimetre of air—a globule about the size of a small marble—there are thirty million trillion molecules. And since the molecule is, as we saw, a group or cluster of atoms, the atom itself is smaller. Atoms, for reasons which we shall see later, differ very greatly from each other in size and weight. It is enough to say that some of them are so small that it would take 400,000,000 of them, in a line, to cover an inch of space; and that it takes at least a quintillion atoms of gold to weigh a single gramme. Five million atoms of helium could be placed in a line across the diameter of a full stop.
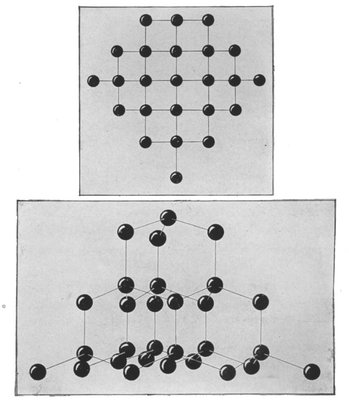
An atom is the smallest particle of a chemical element. Two or more atoms come together to form a molecule: thus molecules form the mass of matter. A molecule of water is made up of two atoms of hydrogen and one atom of oxygen. Molecules of different substances, therefore, are of different sizes according to the number and kind of the particular atoms of which they are composed. A starch molecule contains no less than 25,000 atoms.
Molecules, of course, are invisible. The above diagram illustrates the comparative sizes of molecules.
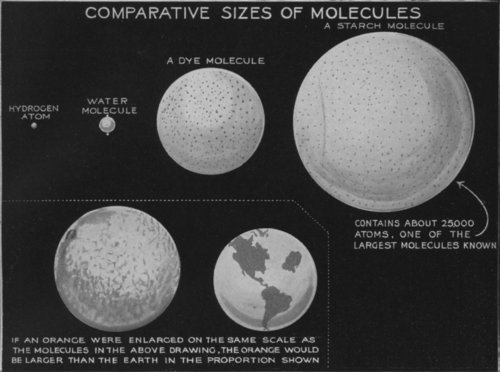
INCONCEIVABLE NUMBERS AND INCONCEIVABLY SMALL PARTICLES
The molecules, which are inconceivably small, are, on the other hand, so numerous that if one was able to place, end to end, all those contained in, for example, a cubic centimetre of gas (less than a fifteenth of a cubic inch), one would obtain a line capable of passing two hundred times round the earth.
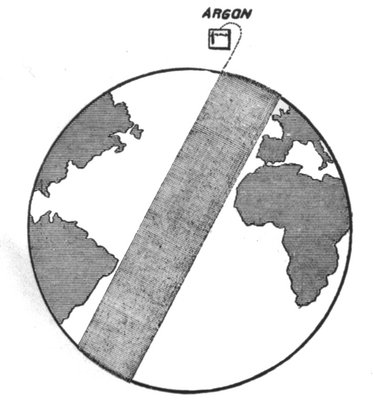
WHAT IS A MILLION?
In dealing with the infinitely small, it is difficult to apprehend the vast figures with which scientists confront us. A million is one thousand thousand. We may realise what this implies if we consider that a clock, beating seconds, takes approximately 278 hours (i.e. one week four days fourteen hours) to tick one million times. A billion is one million million. To tick a billion the clock would tick for over 31,735 years.
(In France and America a thousand millions is called a billion.)
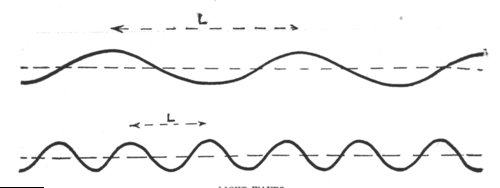
THE BROWNIAN MOVEMENT
A diagram, constructed from actual observations, showing the erratic paths pursued by very fine particles suspended in a liquid, when bombarded by the molecules of the liquid. This movement is called the Brownian movement, and it furnishes a striking illustration of the truth of the theory that the molecules of a body are in a state of continual motion.
The Energy of Atoms
And this is only the beginning of the wonders that were done with "ordinary matter," quite apart from radium and its revelations, to which we will come presently. Most people have heard of "atomic energy," and the extraordinary things that might be accomplished if we could harness this energy and turn it to human use. A deeper and more wonderful source of this energy has been discovered in the last twenty years, but it is well to realise that the atoms themselves have stupendous energy. The atoms of matter are vibrating or gyrating with extraordinary vigour. The piece of cold iron you hold in your hand, the bit of brick you pick up, or the penny you take from your pocket is a colossal reservoir of energy, since it consists of trillions of moving atoms. To realise the total energy, of course, we should have to witness a transformation such as we do in atoms of radio-active elements, about which we shall have something to say presently.
If we put a grain of indigo in a glass of water, or a grain of musk in a perfectly still room, we soon realise that molecules travel. Similarly, the fact that gases spread until they fill every "empty" available space shows definitely that they consist of small particles travelling at great speed. The physicist brings his refined methods to bear on these things, and he measures the[Pg 252] energy and velocity of these infinitely minute molecules. He tells us that molecules of oxygen, at the temperature of melting ice, travel at the rate of about 500 yards a second—more than a quarter of a mile a second. Molecules of hydrogen travel at four times that speed, or three times the speed with which a bullet leaves a rifle. Each molecule of the air, which seems so still in the house on a summer's day, is really travelling faster than a rifle bullet does at the beginning of its journey. It collides with another molecule every twenty-thousandth of an inch of its journey. It is turned from its course 5,000,000,000 times in every second by collisions. If we could stop the molecules of hydrogen gas, and utilise their energy, as we utilise the energy of steam or the energy of the water at Niagara, we should find enough in every gramme of gas (about two-thousandths of a pound) to raise a third of a ton to a height of forty inches.
I have used for comparison the speed of a rifle bullet, and in an earlier generation people would have thought it impossible even to estimate this. It is, of course, easy. We put two screens in the path of the bullet, one near the rifle and the other some distance away. We connect them electrically and use a fine time-recording machine, and the bullet itself registers the time it takes to travel from the first to the second screen.
Now this is very simple and superficial work in comparison with the system of exact and minute measurements which the physicist and chemist use. In one of his interesting works Mr. Charles R. Gibson gives a photograph of two exactly equal pieces of paper in the opposite pans of a fine balance. A single word has been written in pencil on one of these papers, and that little scraping of lead has been enough to bring down the scale! The spectroscope will detect a quantity of matter four million times smaller even than this; and the electroscope is a million times still more sensitive than the spectroscope. We have a heat-measuring instrument, the bolometer, which makes the best thermometer seem Early Victorian. It records the millionth of a degree of[Pg 253] temperature. It is such instruments, multiplied by the score, which enable us to do the fine work recorded in these pages.
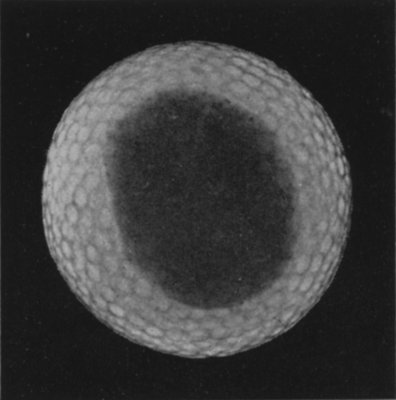
Reproduced from "The Forces of Nature" (Messrs. Macmillan).
A SOAP BUBBLE
The iridescent colours sometimes seen on a soap bubble, as in the illustration, may also be seen in very fine sections of crystals, in glass blown into extremely fine bulbs, on the wings of dragon-flies and the surface of oily water. The different colours correspond to different thicknesses of the surface. Part of the light which strikes these thin coatings is reflected from the upper surface, but another part of the light penetrates the transparent coating and is reflected from the lower surface. It is the mixture of these two reflected rays, their "interference" as it is called, which produces the colours observed. The "black spots" on a soap bubble are the places where the soapy film is thinnest. At the black spots the thickness of the bubble is about the three-millionth part of an inch. If the whole bubble were as thin as this it would be completely invisible.

§ 3